
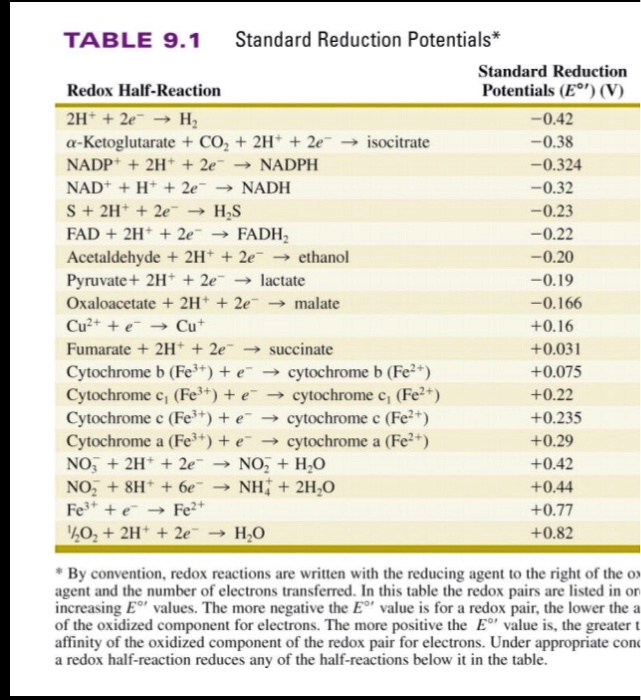
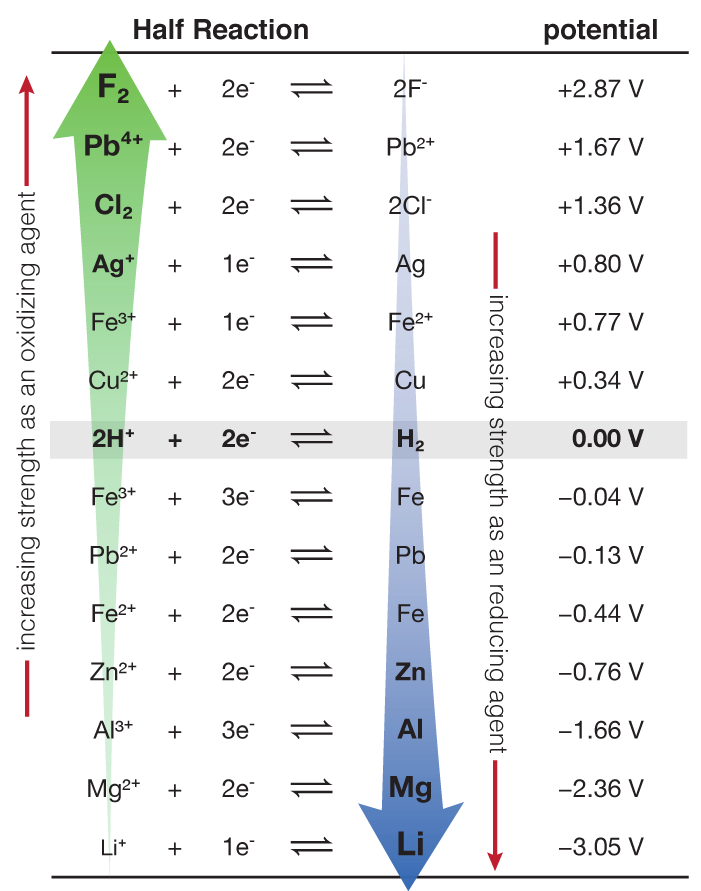
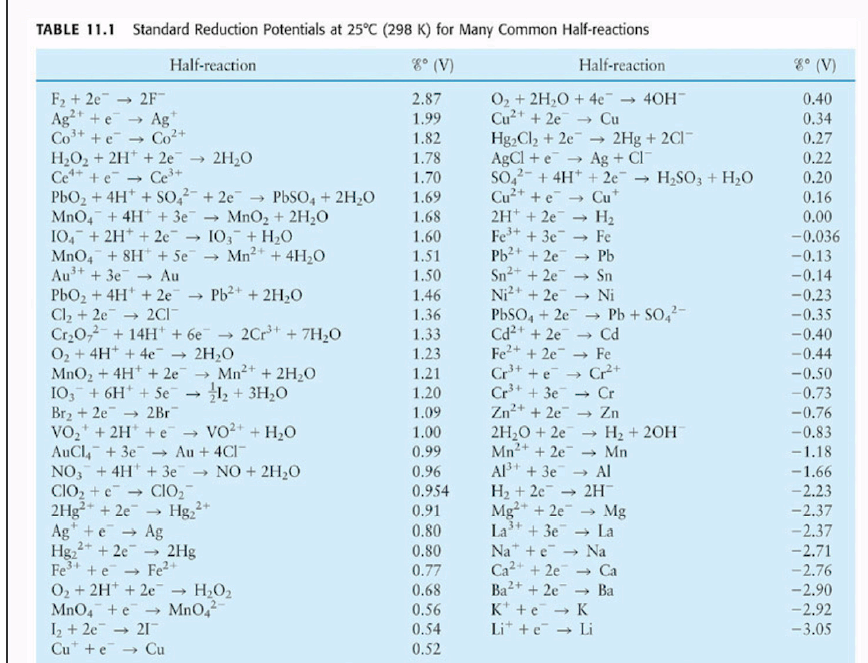
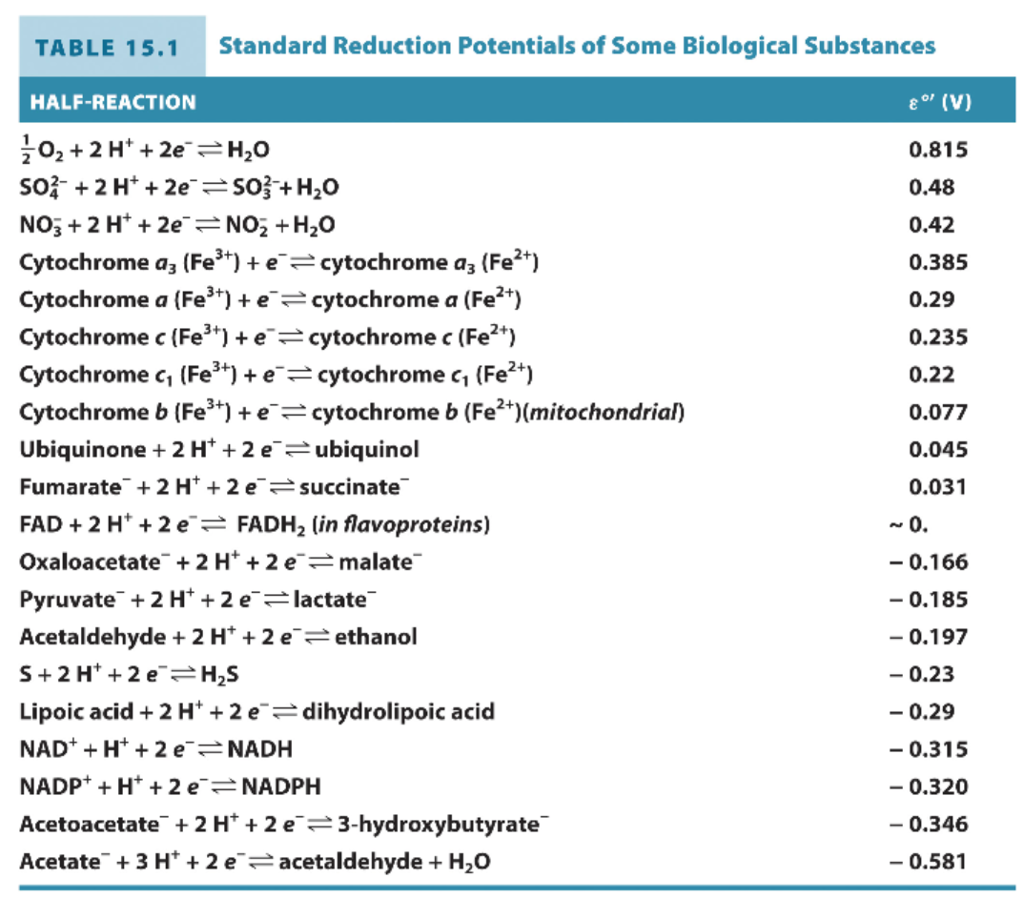
Using the standard electrode potentials given in the table, predict the reaction between the following is possible.Fe^{3+}(aq) and I^{-}(aq)

Study of Reduction-Oxidation Potential and Chemical Charcteristics of a Paddy Field During Rice Growing Season

Table 1 from Tungsten's redox potential is more temperature sensitive than that of molybdenum. | Semantic Scholar

Table 1 from Redox potentials of primary electron acceptor quinone molecule (QA)− and conserved energetics of photosystem II in cyanobacteria with chlorophyll a and chlorophyll d | Semantic Scholar

Table 6-3 from Redox potential and mobility of contaminant oxyanions (As, Sb, Cr) in argillaceous rock subjected to oxic and anoxic cycles | Semantic Scholar












![Redox Potential Values (in volts vs Fc + /Fc) of Borane [B 12 H 12 ] 2−... | Download Table Redox Potential Values (in volts vs Fc + /Fc) of Borane [B 12 H 12 ] 2−... | Download Table](https://www.researchgate.net/publication/310382562/figure/tbl1/AS:668992016498699@1536511534809/Redox-Potential-Values-in-volts-vs-Fc-Fc-of-Borane-B-12-H-12-2-and-Its.png)

